It was 1982 when some colleagues, or I would say friends, followed me in my idea to join forces, convinced like me that only by collaborating would we be able to achieve great results. This was the beginning of GIMEMA, Gruppo Italiano Malattie EMatologiche dell’Adulto (the Italian Adult Haematological Diseases Group), this fantastic adventure that now involves almost all the haematology clinics in Italy
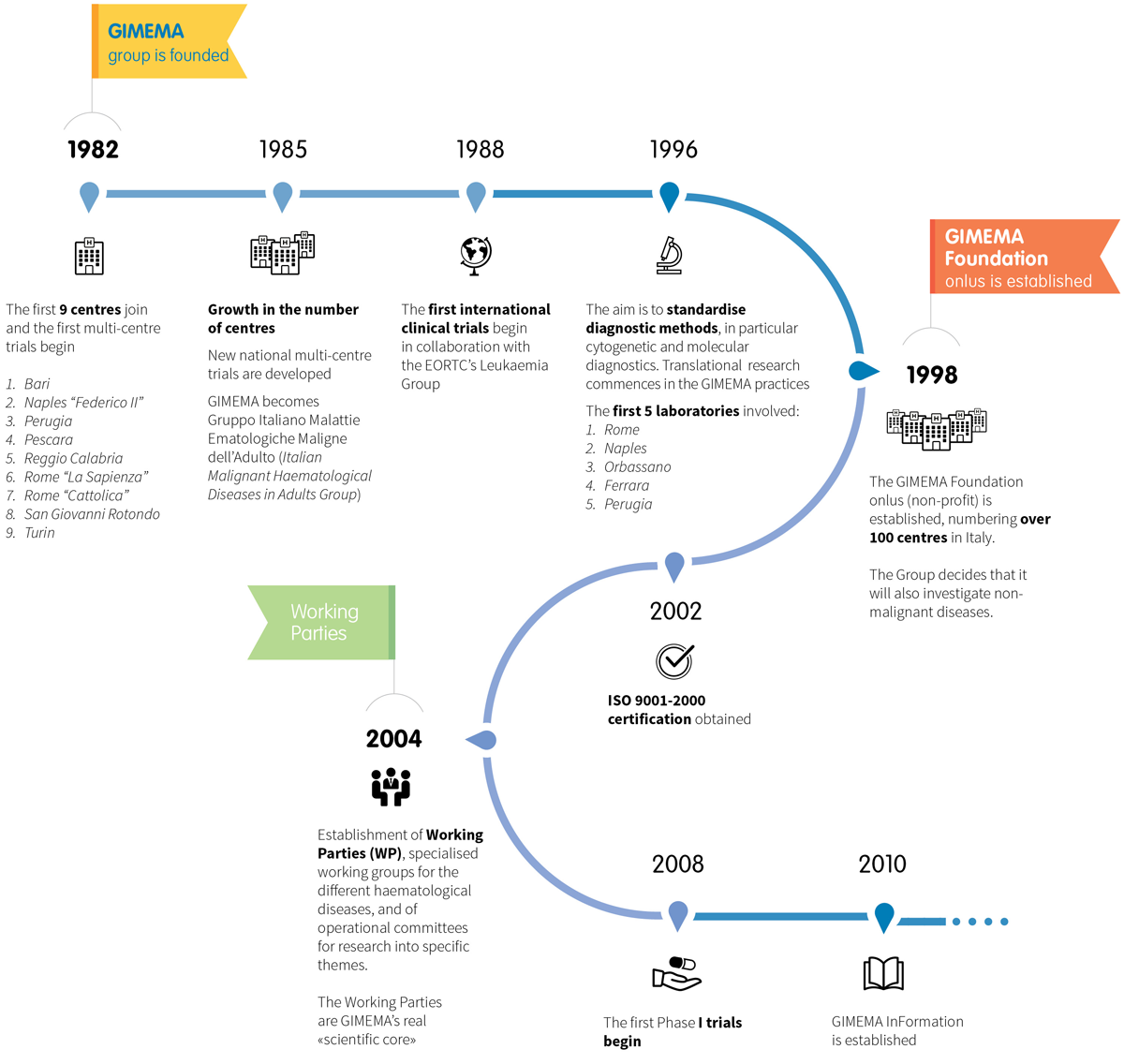
The GIMEMA group, Gruppo Italiano Malattie EMatologiche, is founded, thanks to a proposal from Prof. Franco Mandelli. The first 9 centres join and the first multi-centre trials are started, to assess the validity of the new protocols for diagnosis and treatment in the field of acute leukaemia.
1985
The success of the initiative leads to the establishment of a Data Centre, a central office for the operational and scientific management of the group’s activities.
1988
Formal collaboration between GIMEMA and international research groups begins. In particular, the first testing protocols are implemented with the Leukaemia Group of EORTC(European Organization for Research and Treatment of Cancer).
1996
Start of an ambitious project for the centralisation of biological samples, with the aim of standardising cytogenetic and molecular diagnostic methodologies in particular. Translational research activity effectively begins in the GIMEMA trials.
1998
The GIMEMA Foundation Onlus (non-profit) is established, with the membership of over 100 centres in Italy. Prof. Mandelli is elected as chairman. GIMEMA extends its research into other diseases, also investigating non-malignant conditions.
2002
GIMEMA obtains ISO 9001-2000 certification. GIMEMA is among the first independent research facilities to have certified operational procedures.
2004
The Group’s expansion, the growth in clinical and biological research, the entry into force of European standards and GIMEMA’s increased international visibility, lead to the creation of Working Parties (WP): working groups specialised in the various haematological diseases with operational committees for the elaboration of specific themes.
2008
The first Phase I trials begin. Research is extended to even more innovative medicines, used for the first time in humans.
2010
Establishment of GIMEMA InFormation, the unit responsible for dissemination to the general public , sharing scientific innovations and organising professional training (university Master’s degree and courses).
Today the GIMEMA Foundation is a consolidated body that coordinates a national network with which almost all the haematology centres in Italy collaborate and it manages numerous international collaborative initiatives. The GIMEMA operational centre, the Data centre, is entrusted with the creation of clinical research projects.
The Data Centre is a facility of excellence, certified and recognised internationally for its management of clinical trials.


